Foreword
The phenomenon of luminescence of matter is roughly divided into two categories: one is that the substance is heated to generate heat radiation and the other is that the object is excited by the absorbed energy and transitions to the excited state (unsteady state) in the process of returning to the ground state, with light The form releases energy.
1. Principle of light emission of phosphor
Fluorescence is a process that produces light with little heat compared to thermal radiation. A suitable material absorbs high energy radiation, which in turn emits light, the energy of which is lower than the energy of the excitation radiation. When the luminescent material is a solid, the material is commonly referred to as a phosphor. The high-energy radiation that excites the phosphor may be electrons or ions with high velocity, or photons ranging from gamma rays to visible light.
At present, most of the phosphors actually used for LED applications are powdered photoluminescent phosphors that use blue light (main peak wavelength of 450 nm) as an excitation source. They use isolated electron energy levels of atoms or ions, but pass They combine to form electron transitions between the energy levels of the molecular orbitals.
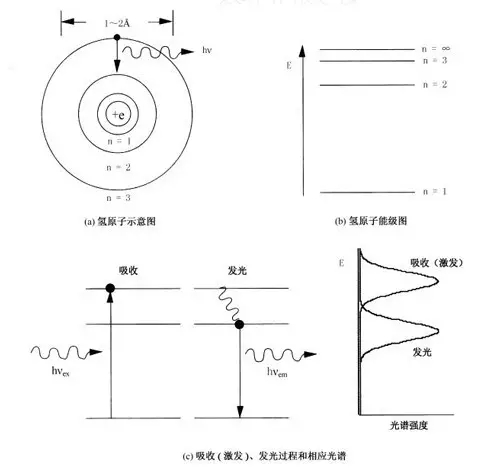
Figure 1-1 Structure of atoms and conversion of light
According to quantum theory, there are multiple energy levels in an isolated single atom or ion. As shown in Figure 1-1(a), when the bound electrons in an atom or ion transition from a high energy level to a low energy level, they form their own intrinsic Glowing. The simplest hydrogen atom will be described below as an example. The hydrogen atom contains one electron, and the electron orbits called 1s, 2s, 3s, ... from the nucleus outward, and each electron orbit corresponds to a different energy level. The one electron of the hydrogen atom is usually located at the innermost side for 1 s. In the orbit, the state of the electron is called the ground state. If the electron is stimulated (excited) by external energy such as electron collision or light, it absorbs the excitation energy and migrates to the outer orbit, such as the 2s orbit. The energy of the 2s orbit is higher than the energy of the 1s orbit. As shown in Figure 1-1(b), this state of the electron is called the excited state. Atomic luminescence is produced when electrons return from the excited state to the ground state (see Figure 1-1(c)).
2. Excitation spectrum of phosphor
At present, the excitation wavelength of LED phosphors is generally between 200nm and 600nm, and the optimum excitation wavelength is generally between 445nm and 460nm, as shown below:
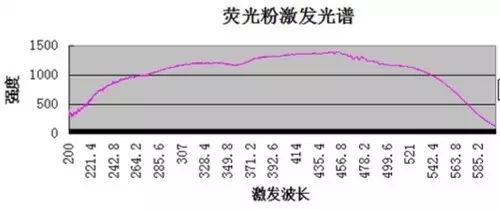
As can be seen from the above figure, the visible wavelength range has a certain excitation effect on the phosphor.
3, fluorescent light efficiency bottleneck
There are many factors that affect the efficacy of the phosphor. Here is a simple analysis of the effect of the phosphor excitation process on the efficacy of the phosphor.
3.1. From the phosphor excitation spectrum, we can see that the wavelength range of light required for the phosphor to be excited to emit photons is very wide, and the excitation efficiency of the wavelength corresponding to the visible light is not much different.
3.2. The half-wave width of the excitation spectrum is too wide, and light of almost the wavelength of the visible light can excite the phosphor.
3.3. Since the excitation spectrum has a half-wave width that is too wide, the visible light that has been excited during the excitation of the phosphor will repeatedly excite the phosphor, and the process is repeated until the light passes through the phosphor.
to sum up
The effect of the phosphor on the excitation process is mainly because the half-wavelength of the excitation spectrum of the phosphor is too wide, that is, almost the wavelength of visible light can excite the phosphor, so that the visible light excited by the phosphor during the excitation process will The phosphor is excited again and the cycle is repeated, and the excitation energy and the excited energy are continuously lost during the repeated cycles, thereby reducing the excitation efficiency of the phosphor. If the phosphor is capable of minimizing the half-wave width of its excitation spectrum as much as possible, the excitation efficiency of the phosphor will be greatly improved.

DBS diode series patch rectifier bridge stack is composed of 4 rectifier diodes connected and packaged in the form of a bridge full-wave rectifier circuit, which belongs to a full bridge. There are four lead-out pins, the connection point of the negative poles of the two diodes is the "positive pole" of the DC output terminal of the full bridge, and the connection point of the positive poles of the other two diodes is the "negative pole" of the DC output terminal of the full bridge. Reverse repetitive peak voltage range: 50V~1000V, average rectified output current 0.8A~1.5A: forward non-repetitive surge current 30A (60Hz sine wave, one cycle, Tj=25°C). For specific parameters, please refer to the specification or consult online customer service.
DB-S diode,DBS Bridge Rectifiers,DBS SMD Bridge Rectifier Diode,Bridge Rectifier Diode DBS
Changzhou Changyuan Electronic Co., Ltd. , https://www.cydiode.com
