Last Monday, in addition to the news of the merger of NXP and Freescale shocked the industry, there is another news that has received much attention - "On March 2, 2015, the world's first batch of 30,000 mass-produced graphene phones were released in Chongqing." According to the news, the graphene mobile phone, the core technology is developed by the Chongqing Institute of Green Intelligent Technology of the Chinese Academy of Sciences, and the Ningbo Institute of Materials Technology and Engineering of the Chinese Academy of Sciences, using the newly developed graphene touch screen, battery and thermal film and other new materials. It has certain advantages in screen display, battery life and preventing the phone from being hot." (behind the first mass production of graphene mobile phones)
This article refers to the address: http://
In the past year, Ren Zhengfei, the founder of Huawei, also praised the prospects of graphene in an interview.
"I think the biggest subversion in this era is the era of silicon in the graphene era, but subversion needs to have inherited development. The success of the silicon era is the most promising leader in the graphene era. The edge opportunity is still the silicon age. The leading company. It is impossible to come out of a small company completely out of thin air, and then lead the pulse of the times, and the development of new technology of graphene in the world is not something that small companies can do."
So, what are the magical aspects of graphene, so that all walks of life are sought after? Let's take a look at what graphene is?
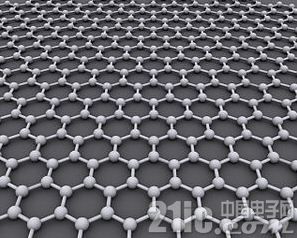
Graphene is formed by carbon atoms in atomic size honeycomb lattice structure. Image Source: Wikipedia
What is graphene?
Graphene is a planar film composed of a carbon atom and a sp2 hybrid orbital to form a hexagonal honeycomb lattice. It has a two-dimensional material with a carbon atom thickness. Graphene has always been considered a hypothetical structure and cannot be stabilized alone until 2004, when the physicists of the University of Manchester, André Heim and Konstantin Novoselov, succeeded in experimenting with graphite. Graphene was isolated, and it was confirmed that it could exist alone. The two also won the 2010 Nobel Prize in Physics for their "creative experiment in two-dimensional graphene materials." [1]
Although the name has the word graphite, it does not depend on the graphite reserves or the characteristics of graphite at all: the graphene is highly conductive, bendable, and mechanically strong, and looks like the future of magical materials. If you open up its list of potential uses—protective coatings, transparent bendable electronic components, ultra-capacitor capacitors, and so on—it's a revolutionary invention. [2]
Graphene is currently the thinnest but hardest nanomaterial in the world. It is almost completely transparent and absorbs only 2.3% of light. The thermal conductivity is as high as 5300 W/m·K, higher than that of carbon nanotubes and diamonds. The electron mobility is over 15000 cm2/V·s, which is higher than that of carbon nanotubes or monocrystalline silicon, and the resistivity is only about 10-6 Ω·cm, which is lower than copper or silver, which is the lowest resistivity in the world. s material. Because of its extremely low resistivity and the extremely fast speed of electronics, it is expected to be used to develop a new generation of electronic components or transistors that are thinner and faster. Since graphene is essentially a transparent, good conductor, it is also suitable for making transparent touch screens, light panels, and even solar cells.
More descriptions [1]
Graphene has the same carbon atom arrangement as the monoatomic layer of graphite, and is a single-layer two-dimensional crystal in which carbon atoms are arranged in a sp2 hybrid orbital in a honeycomb crystal lattice. Graphene is conceivable as an atomic grid formed by carbon atoms and their covalent bonds. The name of graphene comes from the English graphite (graphite) +-ene (end of the olefin). Graphene is considered to be a planar polycyclic aromatic hydrocarbon atom crystal.
The structure of graphene is very stable, and the carbon-carbon bond is only 1.42?. The connection between the carbon atoms inside the graphene is very flexible. When an external force is applied to the graphene, the carbon atom plane is bent and deformed, so that the carbon atoms do not have to be rearranged to adapt to an external force, thereby maintaining structural stability. This stable lattice structure gives graphene excellent thermal conductivity. In addition, when electrons in graphene move in orbit, they do not scatter due to lattice defects or introduction of foreign atoms. Since the interaction between the atoms is very strong, even at the normal temperature, even if the surrounding carbon atoms collide, the interference of the internal electrons of the graphene is very small.
Graphene is the basic unit constituting the following carbon allotropes: graphite, charcoal, carbon nanotubes and fullerenes. The perfect graphene is two-dimensional, it only includes hexagons (equal hexagons); if there are pentagons and heptagons, it will constitute a defect of graphene. Twelve pentagonal graphenes together form fullerenes.
Graphene is rolled into a barrel shape and can be used as a carbon nanotube; in addition, graphene is also made into a ballistic transistor and has attracted the interest of a large number of scientists. In March 2006, researchers at the Georgia Institute of Technology announced that they successfully fabricated graphene planar field-effect transistors and observed quantum interference effects. Based on this result, a graphene-based circuit was developed.
The advent of graphene has caused a worldwide research boom. It is the thinnest of the known materials, the material is very strong and hard, and at room temperature, the electrons are transmitted faster than known conductors.
It has been around for ten years, but where is the transparent mobile phone?[2]
In fact, in 2012, Konstantin Novoselov, who won the Nobel Prize for graphene, and his colleagues once published an article on Nature to discuss the future of graphene, two years of development. They also basically proved their predictions. He believes that as a material, graphene "the future is bright, the road is tortuous", although it may play a major role in the future, but this scene will not come before overcoming several major difficulties. More importantly, considering the huge cost of industrial renewal, the benefits of graphene may not be enough to simply replace existing equipment – ​​its real future may be a new application tailored to its unique characteristics. .
Preparation method [1]
In 2008, graphene prepared by mechanical stripping was one of the most expensive materials in the world, and a small sample of human hair cross-section size cost $1,000. Gradually, with the scale of the preparation process, the cost is much lower. Now, company lines can buy and sell graphene in metric tons. On the other hand, the price of the graphene film grown on the surface of the silicon carbide is mainly determined by the substrate cost, which was about $100/cm2 in 2009. Using chemical vapor deposition, carbon atoms are deposited on a nickel metal substrate to form graphene, which is etched away from nickel metal and then converted and deposited onto other substrates. Thus, a graphene film having a size of 30 inches wide can be produced more inexpensively.

The Novoselov team donated graphite, graphene and tape to Stockholm. The signature "Andre Geim" on the tape is the one who won the Nobel Prize with Novoselov. Image source: wikipedia
Tear tape method / slight rubbing method
The most common is the micromechanical separation method, which directly cuts the graphene sheets from the larger crystals. In 2004, Heim et al. used this method to prepare a single layer of graphene, which can be stably existed in the external environment. A typical preparation method is to use another material to puff or introduce defective pyrolytic graphite to rub. The surface of the bulk graphite produces flake-like crystals, and the flake-like crystals contain a single layer of graphene. However, the disadvantage is that this method utilizes a sheet obtained by rubbing the graphite surface to screen a single layer of graphene sheet, the size of which is difficult to control, and it is impossible to reliably produce a graphite sheet sample for the length of the supply.
Epitaxial growth of silicon carbide surface
In this method, silicon is removed by heating single crystal silicon carbide, and a graphene sheet layer is decomposed on a single crystal (0001) plane. The specific process is: the sample obtained by etching with oxygen or hydrogen is heated by electron bombardment under high vacuum to remove oxides. After using Auger electron spectroscopy to confirm that the oxide on the surface is completely removed, the sample is heated to raise the temperature to 1250~1450 °C and then the temperature is 1 min~20 min, thus forming a very thin graphite layer. After several years of exploration, Claire Berger et al. have been able to controllably prepare single or multiple layers of graphene. It is relatively easy to obtain up to 100 layers of multilayer graphene on a C-terminated surface. The thickness is determined by the heating temperature, and it is difficult to prepare a graphene having a single thickness in a large area.
Metal surface growth
The orientation epitaxy method uses the growth matrix atomic structure to "speculate" graphene. First, the carbon atoms are infiltrated into the crucible at 1150 ° C, and then cooled. After cooling to 850 ° C, a large amount of carbon atoms absorbed before will float to the surface of the crucible. A single layer of carbon atoms "islands" in the shape of the lens fill the entire surface of the substrate, and eventually they can grow into a complete layer of graphene. After the first layer covers 80%, the second layer begins to grow. The underlying graphene will have a strong interaction with the ruthenium, while the second layer will be almost completely separated from the ruthenium, leaving only weakly coupled, and the resulting single-layer graphene sheet will perform satisfactorily. However, the graphene sheets produced by this method tend to be uneven in thickness, and the adhesion between the graphene and the matrix affects the characteristics of the carbon layer. In addition, the substrate used by Peter Sutter et al. is a rare metal ruthenium.
Oxidation thinned graphite sheet method
Graphene can also be thinned by layering of graphite sheets by heating and oxidation to obtain single- and double-layer graphene.
肼 reduction method
A graphene oxide paper is placed in a pure hydrazine (Hydrazine, N2H4) solution (a compound of a hydrogen atom and a nitrogen atom), which reduces the graphene oxide paper to a single layer of graphene.
Sodium oxychloride lysis
A paper published in 2008 describes a procedure that can produce graphene in grams. First, the ethanol is reduced with sodium metal, and then the obtained ethoxide product is cleaved, and the sodium salt is removed by water washing to obtain a graphene which is adhered together, and then oscillated by mild sonication to form a gram. The amount of pure graphene.
Cutting carbon nanotube method
Cutting carbon nanotubes is also an experimental method for making graphene ribbons. One of the methods uses potassium permanganate and sulfuric acid to cut multi-walled carbon nanotubes in solution. Another method uses plasma etching of a portion of the nanotubes embedded in the polymer.
Sonic processing of graphite
This method comprises graphite dispersed in a suitable liquid medium and then ultrasonically treated. The non-expanded graphite is finally separated from the graphene by centrifugation. This method was first proposed by Hernandez et al., who obtained a graphene concentration of 0.01 mg/ml in N-methylpyrrolidone (NMP). This method was then primarily improved by multiple research groups. In particular, it has been greatly improved by the team of Alberto Mariani in Italy. Mariani et al. reached a concentration of 2.1 mg/ml in NMP (the highest in the solvent). The highest concentration of graphene published by the same group is reported in any liquid to date and obtained by any method. An example is the use of a suitable ionizing liquid as a dispersion medium for graphite stripping; a very high concentration of 5.33 mg/ml is obtained in this medium.
Some recent applications
Graphene-based flexible display (Flexible Display) [3]
Text / Paul Buckley
The Cambridge Graphene Centre (CGC) and Plastic Logic have announced that they will first apply graphene to transistor-based flexible devices, a move that will open up opportunities for fully wearable and flexible devices.
The partnership between the two organizations allows the Cambridge Graphite Center (CGC) expertise in graphene to be combined with the logic and display processing processes that Plastic Logic has developed for flexible electronics. This prototype is the first example of how such a partnership will accelerate the commercial development of graphene, and has taken the lead in the development of more graphene and graphene-like materials for flexible electronics. step.
The prototype is an active-matrix electrophoretic display similar to that used in today's e-readers, but it is made of flexible plastic instead of glass. The pixel electronics, or backplane, of the display, including a solution-processed graphene electrode, replaces the sputtered metal in the traditional equipment of Plastic Logic, compared to conventional displays. The electrode layer benefits both the product and the process.
Graphene has better flexibility than conventional ceramic alternatives such as indium tin oxide (ITO) and also has better permeability than metal films. This ultra-flexible graphene layer enables many products, including foldable electronics. Graphene can also be treated with a solution, which brings inherent advantages in more efficient printing and roll-to-roll manufacturing methods.
The backplane with 150 pixels per inch is fabricated at low temperatures using Plastic Logic's Organic Thin Film Transistor (OTFT) technology. The graphene electrode is deposited in solution, followed by patterning on micron-scale features and then completing the backsheet.
For this prototype, the backplane incorporates an electrophoretic imaging film to develop a display with ultra-low power and durability. Future demonstrations may incorporate liquid crystal display (LCD) and organic light emitting diode (OLED) technologies to achieve full color and video functionality. The lightweight and flexible active matrix backplane can be used for sensing, while novel digital medical imaging and gesture recognition applications are already under development.
Professor Andrea Ferrari, Director of the Cambridge Graphene Center, explained: “We are pleased to see our collaboration with Plastic Logic to obtain the results of the first graphene-based electrophoretic display made from graphene in its pixel electronics. This is an important step towards achieving a fully wearable and flexible device that reinforces the cluster of Cambridge graphene technology and demonstrates the developments that have helped bring graphene from the laboratory to the plant. The key role of effective industry-university cooperation in this."
Indro Mukerjee, CEO of Plastic Logic, said: "The potential of graphene is well known, but industrial process engineering now requires that graphene be brought from the laboratory to the industry. This demonstration highlights the development of Plastic Logic. The trend's leading position, and this trend will soon enable a new generation of ultra-flexible, or even foldable, electronic products."
This program is jointly funded by the Engineering and Physical Sciences Research Council (EPSRC) and the EUs Graphene Flagship.
Transparent sensor that provides a better view of brain activity [3]
Text / Amy Norcross
A team of researchers from the University of Wisconsin-Madison has been developed with the support of the Defense Advanced Research Projects Agency's (DARPA) Reliable Neural-Interface Technology (RE-NET) program. An array of "invisible" implantable medical sensors that will not obstruct the observation of brain activity.
According to a recent article published on Phys.org, "Electrical monitoring and stimulation of neural signals is the only technology that can be relied upon to study brain function, while emerging optical techniques using photons rather than electrons are nerves. The visualization of the network structure and the exploration of brain functions have opened up new opportunities. Electrical and optical technologies have obvious complementary advantages. If they are used together, they will provide far-reaching benefits for brain research in high resolution. However, combining these technologies is a challenging task because traditional metal electrode technology is too thick (>500 nm) to make light inaccessible, making them incompatible with many optical methods."
Justin Williams, professor of biomedical engineering and neurosurgery at the University of Wisconsin-Madison, said: "A holy grail of nerve implant technology is that we really want an implantable device that does not interfere with any traditional imaging diagnostics. Traditional implants The technology looks like a square of dots, you can't see anything under it. We want to make a transparent electronic device."
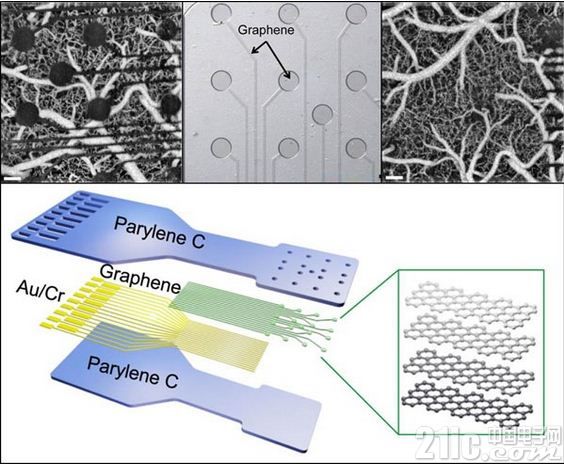
The traditional metal electrode technique (top left) blocks the view of the nerve tissue. The new graphene sensor technology developed by DARPA's RE-NET program is electrically conductive and is only 4 atoms thick, hundreds of times thinner than current contacts (top). This extremely thin thickness allows almost all light to travel across a wide range of wavelengths. Placed in a flexible plastic-lined sensor (bottom) that conforms to the shape of the tissue is part of a proof-of-concept tool that exhibits smaller, more translucent contacts that can be used both electrically and optically. Measurement and stimulation of nerve tissue (top right). Data source: DARPA.
Due to the elasticity and softness of graphene and its good electrical conductivity, it was chosen as the material for the new sensor. And it is also non-toxic to biological systems. Zhenqiang (Jack) Ma, a professor of electrical and calculator engineering at the University of Wisconsin-Madison, points out that the material requirements are thin enough and strong enough to survive in the body. Graphene placed on a flexible plastic that conforms to the shape of the tissue (bottom) "has the best balance between transparency, strength and electrical conductivity". This graphene sensor is only 4 atoms thick, and this extremely thin thickness allows almost all light to travel across a wide range of wavelengths, from ultraviolet to deep infrared.
Doug Weber, DARPA's project manager, said, "This technology demonstrates the potential for breakthroughs in visualizing and quantifying neural network activity in the brain. It also measures electrical activity at a wide range and at a rapid rate, and provides neural network anatomy. This ability to directly visualize and modulate provides unprecedented insight into the relationship between brain structure and function, and more importantly, how these relationships develop over time or are damaged Or the trouble of the disease."
Applications of this technology include the nervous system, cardiac monitoring, and even contact lenses. The team at the University of Wisconsin-Madison, in collaboration with researchers at the University of Chicago in Illinois, developed a prototype of a contact lens that includes dozens of invisible sensors that can be used to detect the retina Damaged situation. The University of Chicago, Illinois, is also developing a method for early diagnosis of glaucoma.
According to Kip Ludwig, director of neuroengineering at the Institute of Neurological Disorders and Stroke, another area of ​​application where transparent sensors can benefit is neuromodulation therapy, and more and more doctors use neuromodulation to treat high blood pressure. Epilepsy and Parkinson's disease patients control symptoms, restore function and relieve pain. "Although significant improvements can be seen in the neuromodulation clinical trials of these diseases, we are still at an early stage of how these therapies work and our ability to improve existing or new treatments," he said. â€
Ludwig added that researchers' current capabilities are limited for direct observation of how the body produces electrical signals and how it reacts to externally generated electrical signals. He said: "The combination of clear electrodes and advanced optogenetics and photovoltage probe technology will allow researchers to isolate those biological mechanisms. This basic knowledge can be used for existing neuromodulation. Significant improvements in treatment and the identification of new treatments have a catalytic effect."
Aluminum Electrolytic Capacitors/ Ceramic Capacitors
Aluminum Electrolytic Capacitors/ Ceramic Capacitors
Aluminum Electrolytic Capacitors,Electrolytic capacitor,Ceramic Capacitor
YANGZHOU POSITIONING TECH CO., LTD , https://www.yzpstcc.com
